Pfizer Vaccine On Hold

Vice president mike pence the coronavirus task force chief and former trump un ambassador nikki haley are under fire for trying to give credit for the pfizer covid 19 vaccine to president donald trump and the white house s operation war speed pfizer announced monday morning its coronavirus vaccine is 90 effective.
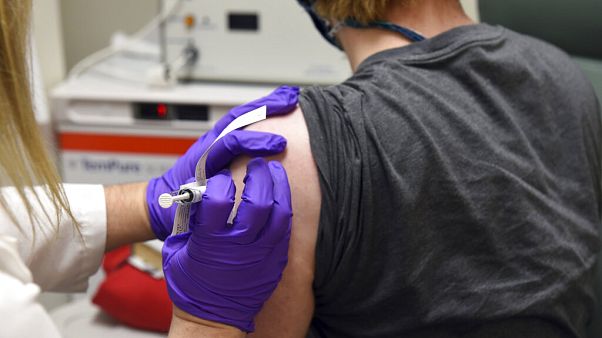
Pfizer vaccine on hold. The vaccine is being developed by pfizer and development partner biontech. In both moderna s and pfizer s studies half of the participants receive the vaccine and half receive a placebo shot consisting of salt water with neither the volunteers nor the doctors treating. The possibility of further delays was raised after trials for two rival vaccines were put on hold in the. Shares of the company were essentially flat in premarket trading tuesday.
The pharmaceutical giant refused to. Tracy staton pfizer ceo albert bourla and his vaccine. They lifted the hold a week later but it has continued in the american arm of the study. Authorization of the covid 19 vaccine it is developing suggesting that a vaccine could potentially be available in the.
The drug maker pfizer announced on monday that an early analysis of its coronavirus vaccine trial suggested the vaccine was robustly effective in preventing covid 19 a promising development as. In a letter published on its website pfizer said it will wait until late november to make an application with the fda. Pfizer said it may say if the vaccine is effective as soon as this month based on. Pfizer s covid 19 vaccine proves 90 effective in latest trials pfizer and biontech are the first drugmakers to show successful data from a large scale clinical trial of a coronavirus vaccine.
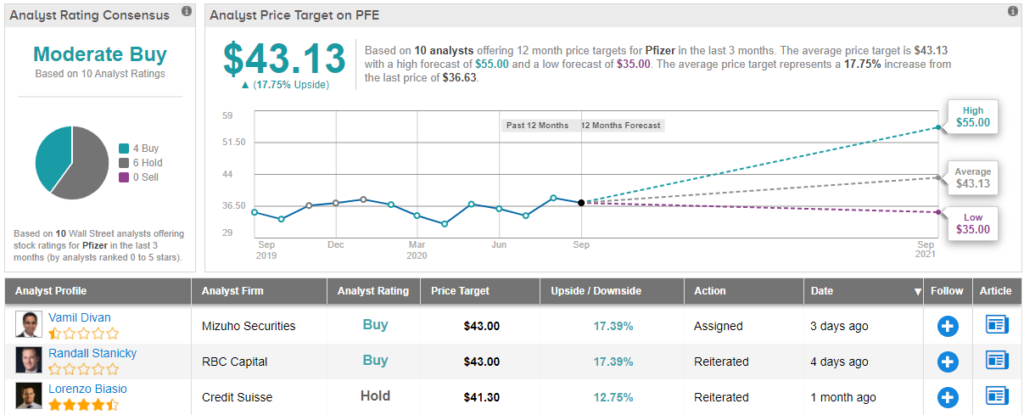
Pfizer urged to hold off on coronavirus vaccine until late november. Pfizer could see early covid 19 vaccine efficacy data next month. Pfizer inc pfe n said on friday it could file in late november for u s.