Covid Vaccine Failed Phase 3
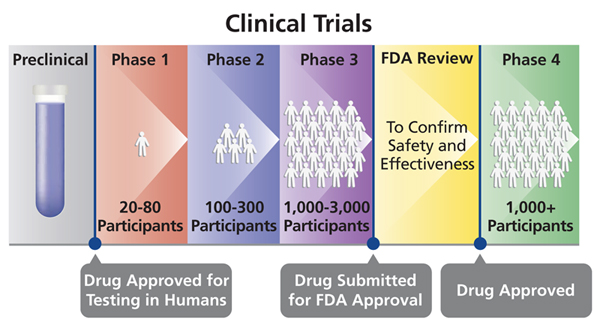
Previous vaccines for other diseases have looked successful in phases 1 and 2 but have failed in phase 3.
Covid vaccine failed phase 3. With more than 200 000 americans dead from covid 19 pharmaceutical companies are racing to roll out vaccines to halt the coronavirus pandemic. The phase 3 trials of indigenously developed coronavirus vaccine covaxin were deferred by almost 15 days at sanjay gandhi post graduate institute of medical sciences in lucknow. All vaccines preclinical phase 1 phase 2 phase 3 approved genetic vaccines vaccines that deliver one or more of the coronavirus s own genes into our cells to provoke an immune response. The covid 19 vaccine being developed by pfizer and its partner biontech has shown to be effective blocking infection in 90 percent of participants in its phase 3 clinical trial the companies.
A senior official at the world health organization who on thursday confirmed that six experimental coronavirus vaccines including three candidates from china have entered phase 3 clinical trials. Russia has approved a covid 19 vaccine called sputnik v without finishing phase 3 trials. Signage is seen on july 22 2020 in new york city. A potential coronavirus cure has failed to meet the primary goals of its phase 3 trial despite the promise the drug has shown elsewhere.
Covid 19 vaccine trials are now in phase 3 studies. Pfizer says covid 19 vaccine 90 effective in phase 3 trial monday 9 november 2020 2 30pm pfizer inc. Dr michael ryan executive director of the who health. The results of phase 2 trials of covaxin are still being evaluated by the drugs controller general of india and hence now the next phase trials are expected to start in the last week of october 2020.
Paris afp bloomberg a vaccine jointly developed by pfizer and biontech was 90 per cent effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday.