Covid Vaccine Efficacy Data
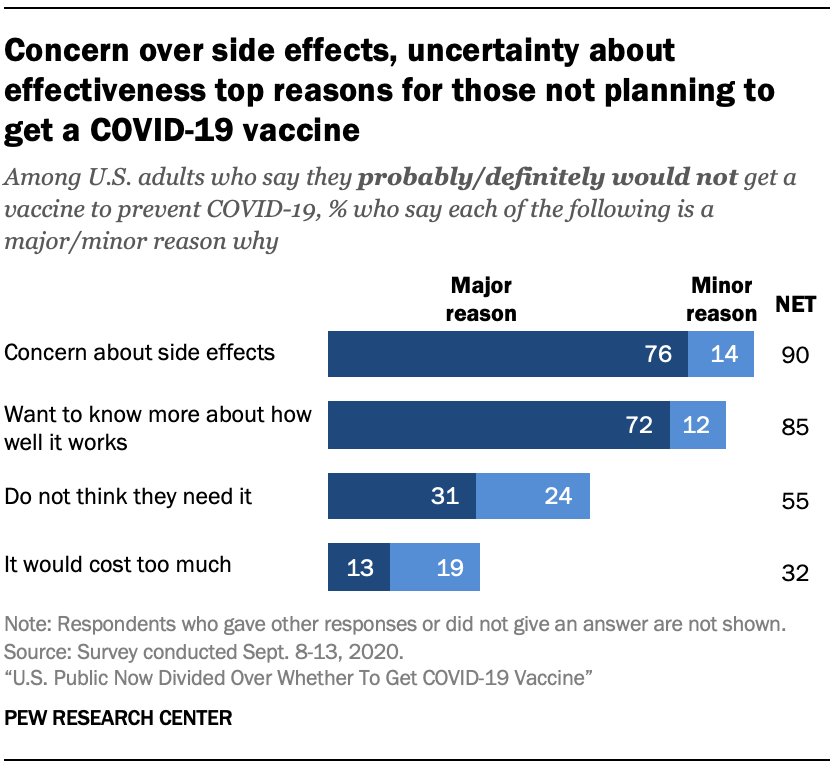
Early analysis suggests this vaccine has an efficacy of over 90.
Covid vaccine efficacy data. The first set of results from our phase 3 covid 19 vaccine trial provides the initial evidence of our vaccine s ability to prevent covid 19 dr. Dow futures pointed to about a 6 rally at monday s open after pfizer pfe and biontech bntx said that data from their covid 19 vaccine trial showed 90 efficacy. So if you took ten people who were going to get sick from covid 19 and vaccinated them only one would get sick. They found no serious adverse effects and measured an.
The vaccine is so far 90 percent effective. Pfizer is the first drug company to release data from a large phase 3 trial for a coronavirus vaccine designed to show both effectiveness and safety. Why a covid 19 vaccine that s. Albert bourla pfizer chairman and ceo said in a.
As cases of coronavirus in the u s. Preclinical data for covid 19 vaccine candidate show effectiveness and advantages. Other open questions about the rapidly developed covid 19 vaccines include long term safety indicating the critical need for pharmacovigilance activities the duration of vaccine protection the efficacy of a partial vaccination series or of lower doses the vaccine s level of protection against severe infection and death efficacy by baseline serostatus and the potential for the virus. They have vaccines in clinical trials for a number of diseases and in june they announced interim data from a phase 1 trial on covid 19.
Phase 1 data shows that. The broad surge in stocks. By jeff hansen university of alabama at birmingham.