Coronavirus Vaccine Zydus Cadila
.jpg)
The pharmaceutical company has received approval from the dcgi to conduct human trials for the coronavirus vaccine it has named zycov d.
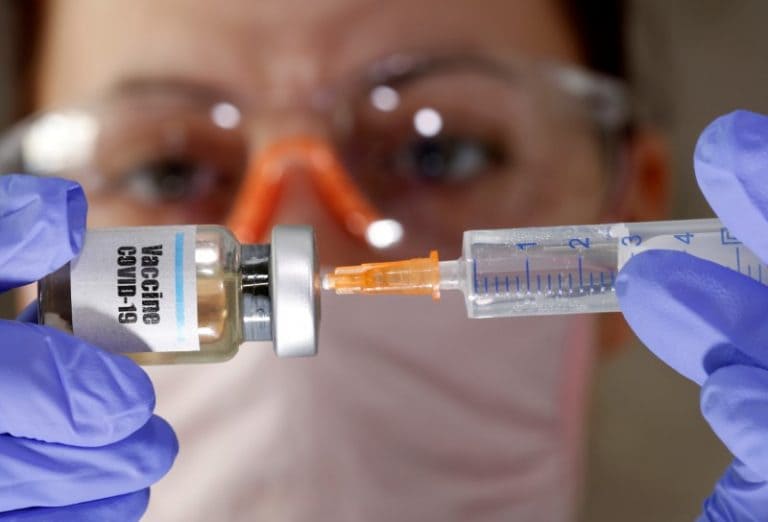
Coronavirus vaccine zydus cadila. Zydus cadila on friday announced that its plasmid dna vaccine candidate for covid 19 zycov d has received permission from drug controller general of india central drugs standard control. For zycov d which we aim to complete in three months and meet the regulator for their guidance. Covid 19 sequence published zydus initiates fast track. A novel approach for covid 19 vaccine development with.
Zydus cadila launches a fast tracked programme to develop vaccine for the novel coronavirus 2019 ncov covid 19 adopting a two pronged approach a dna based vaccine and a live attenuated recombinant measles virus vectored vaccine to combat the virus ahmedabad india 15 february 2020. Zydus cadila an innovationdriven global pharmaceutical. Cadila healthcare zydus cadila said it has completed enrolment and dosing of 1 000 volunteers in phase 2 clinical trials of its potential covid 19 vaccine zycov d and plans to submit the data. India s second coronavirus vaccine by zydus cadila gets dcgi nod for human clinical trials the approval process was fast tracked following a recommendation by the subject expert committee on covid.
The trials will begin this month with 1 000. Dcgi dr v g somani has given approval for the phase i and ii clinical trials on humans of the potential novel coronavirus vaccine developed by zydus cadila healthcare ltd on thursday after its. 5 zycov d vaccine. Icmr bharat biotech s covaxin zydus cadila s zycov d are making progress as more than 140 vaccine candidates around the world are at several.
Indian pharmaceutical company zydus cadila will begin the phase 2 clinical trials of its experimental coronavirus vaccine on thursday. The ahmedabad based firm on wednesday said that its dna based vaccine zycov d developed against the sars cov 2 the virus that causes covid 19 was found to be safe and well tolerated in phase 1 trial. The company s teams in india and europe will leverage two different methods to develop the vaccine.