Coronavirus Vaccine Phase 3
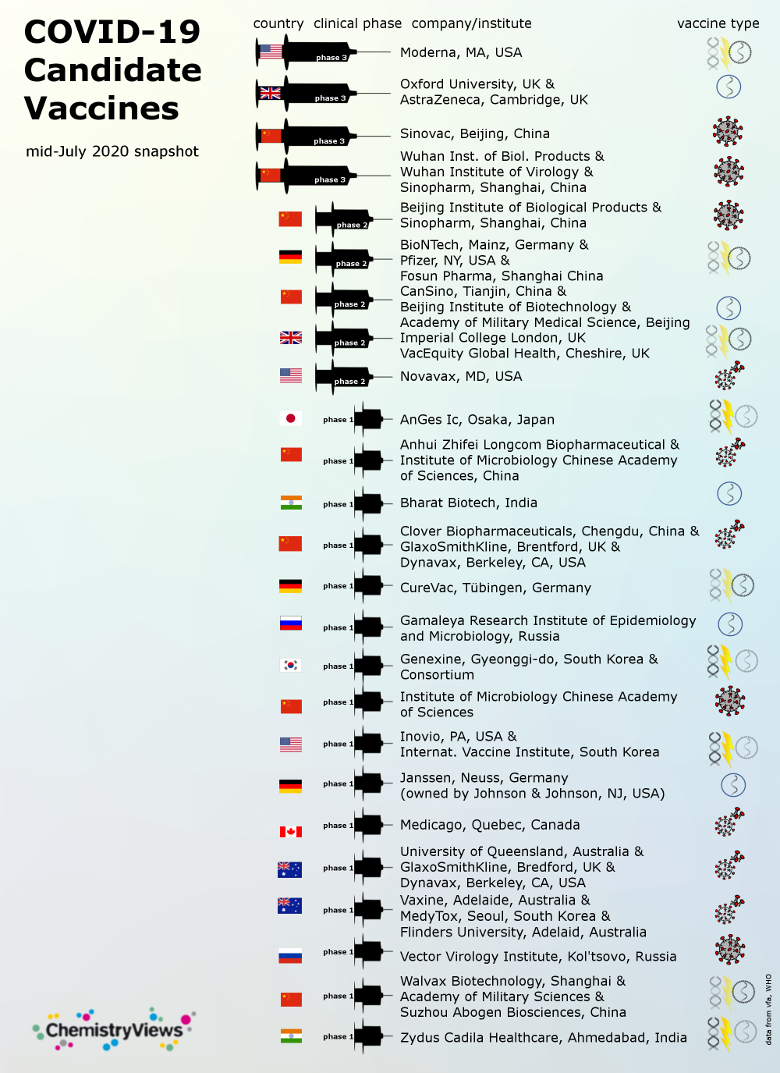
A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun.
Coronavirus vaccine phase 3. The vaccine currently in phase iii of its trials which. A vaccine jointly developed by pfizer and biontech was 90 effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday. Azd1222 developed by astrazeneca nasdaq azn and the university of oxford. Here is a look at how recipients feel about the covid 19 vaccines and what they went through.
The statement was released as. Researchers and public health experts including in india are hopeful that one or several experimental coronavirus vaccines will be ready by the end of this year or early next year. The university of oxford astrazeneca backed novel coronavirus vaccine has been one of the leading vaccine candidates in the global race. This phase of the trial is expected to involve 30 000 volunteers and will test whether the vaccine protects people against the coronavirus.
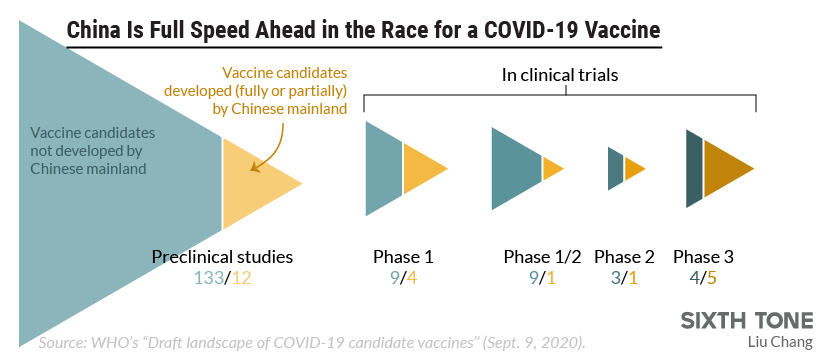
The vaccine is expected to enter phase 3 testing next week. The novel coronavirus pandemic has triggered an unprecedented global research effort to find a safe and effective vaccine against the sars cov 2 the virus that causes covid 19. Currently only three experimental coronavirus vaccines are in phase 3 clinical trials being conducted in the u s. More than 60 000 chinese citizens have received the potential vaccines in phase 3 trials.
The first phase 3 coronavirus vaccine trial results are here and they re incredible a medical professional is about to administer a drug via injection to a patient.