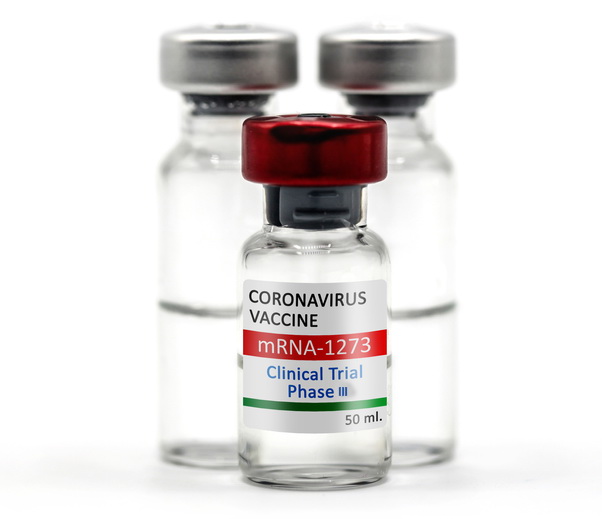
Coronavirus Vaccine Phase 3 Moderna
Phase 3 moderna develops vaccines based on messenger rna mrna to produce viral proteins in the body.
Coronavirus vaccine phase 3 moderna. Moderna a front runner in the covid 19 vaccine race said last week it had completed enrollment for its 30 000 participant late stage. Shares of moderna were up more than 3 in midmorning trading. Moderna s coronavirus vaccine entered a new and crucial phase of testing on monday. Its phase 3 clinical trial which involves 30 000 volunteers at 87 sites across the us.
Moderna has finalized the design and dosage of its phase 3 covid 19 vaccine trial keeping it on track to start the pivotal test next month. The company s early stage testing continues. Racing ahead with its candidate mrna 1273 the biotech has now finished enrolling the 30 000 participants needed for its late stage trial. Biotech company moderna said on monday that it has started dosing participants in the phase iii clinical trial for its coronavirus vaccine candidate.
Moderna is one step closer to a covid 19 vaccine. Moderna has finalized plans for phase 3 testing of its covid 19 vaccine to start and july and include 30 000 participants. Moderna is charging between 32 and 37 per dose for its coronavirus vaccine for some customers under. A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun.
Anthony fauci said results from early. Mrna has outlined plans for phase 3 clinical trials for its covid 19 vaccine candidate mrna 1273 which will begin in july and is expected to include about 30 000 participants.








.jpg)






