Coronavirus Vaccine Moderna Phase 3 Results

In january they began developing a vaccine for the.
Coronavirus vaccine moderna phase 3 results. Moderna s covid 19 vaccine candidate is finally going to enter the final phase of human trials on july 27 as it has shown positive results in previous human trials. Mrna initiated a large scale phase 3 study of its coronavirus vaccine candidate in mid july an analyst at jefferies conducted a statistical analysis of the study design to. Phase 3 moderna develops vaccines based on messenger rna mrna to produce viral proteins in the body. Moderna is one step closer to a covid 19 vaccine.
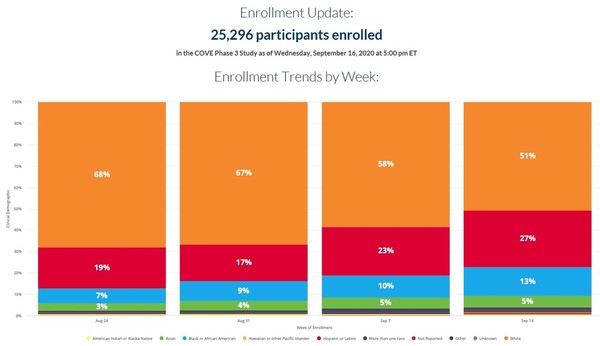
Et to discuss the results. July 15 2020 12 10 pm ist. Racing ahead with its candidate mrna 1273 the biotech has now finished enrolling the 30 000 participants needed for its late stage trial. Moderna s coronavirus vaccine entered a new and crucial phase of testing on monday.
A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun. Its phase 3 clinical trial which involves 30 000 volunteers at 87 sites across the us. The first set of results from our phase 3 covid 19 vaccine trial provides the initial evidence of our vaccine s ability to prevent covid 19 pfizer ceo albert bourla said in a statement. Tony potts a 69 year old retiree living in ormond beach fla is a participant in a phase 3 covid 19 vaccine clinical trial sponsored by moderna.
Supplied a phase 1 study typically studies a small number of people and focuses on whether a vaccine is safe and. The company will hold a conference call at 4 30 p m. The vaccine from moderna is one of several in development to fight the coronavirus which has infected more than 23.






.jpg)




