Coronavirus Vaccine Moderna Phase 2

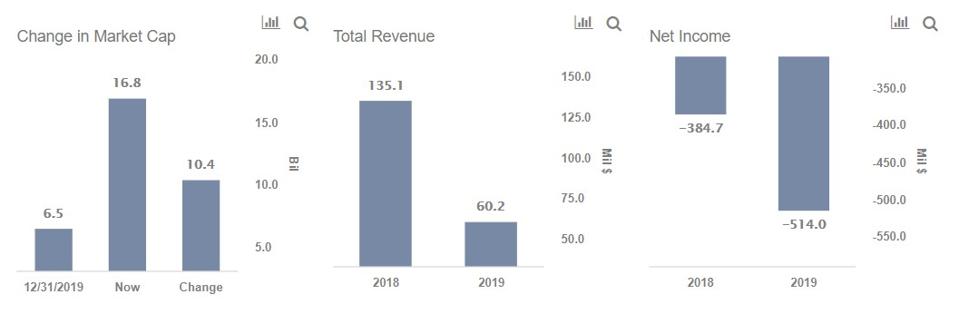
8 2020 moderna inc nasdaq.
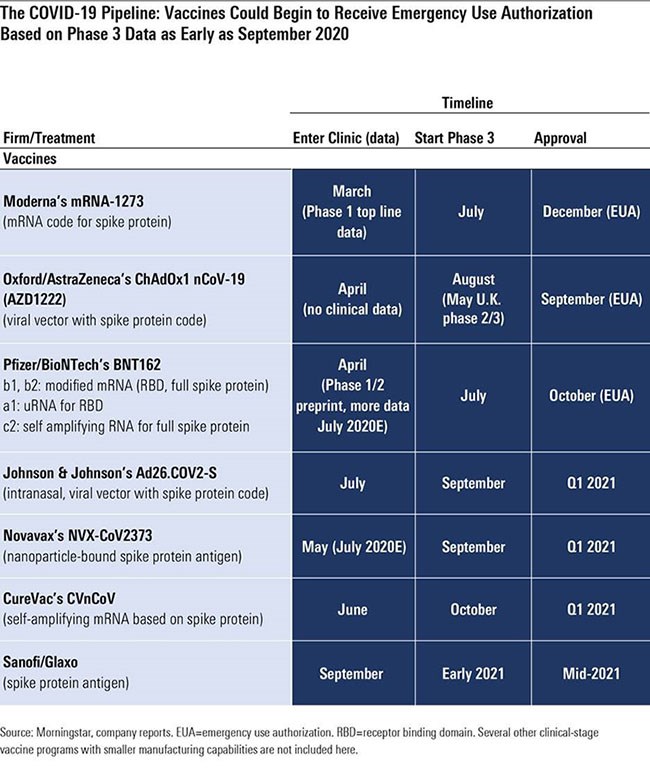
Coronavirus vaccine moderna phase 2. Mrna announced late friday it has dosed the first participants in a phase 2 trial of its mrna vaccine codenamed mrna 1273 against sars cov 2 the virus that causes covid 19. Moderna completes enrollment for phase 2 coronavirus vaccine trial protocol for the phase 3 study has already been finalized following feedback moderna received from the us food and drug. Note that our tracker counts a combined phase 1 2 trial as. Government by alice park may 12 2020 3 20 pm edt.
May 30 2020 the drug company moderna has entered phase two in its search for a coronavirus vaccine by giving the vaccine to the first of 600 participants the company said friday in a news release. Phase 1 human trials of the potential vaccine began in the seattle area. Moderna has started dosing the first participants in all age cohorts of the phase ii clinical trial of its covid 19 vaccine candidate mrna 1273. Mrna said monday that it has submitted an investigational new drug application to the fda seeking the regulatory agency s consent to proceed with phase 2 and late stage.
Some coronavirus vaccines are now in phase 1 2 trials for example in which they are tested for the first time on hundreds of people. Moderna s covid 19 vaccine moves to phase 2 testing and gets fast track approval from the u s. The food and drug administration fda has given approval to biotech company moderna to begin the next phase of testing on its coronavirus vaccine candidate the company announced thursday may 7.