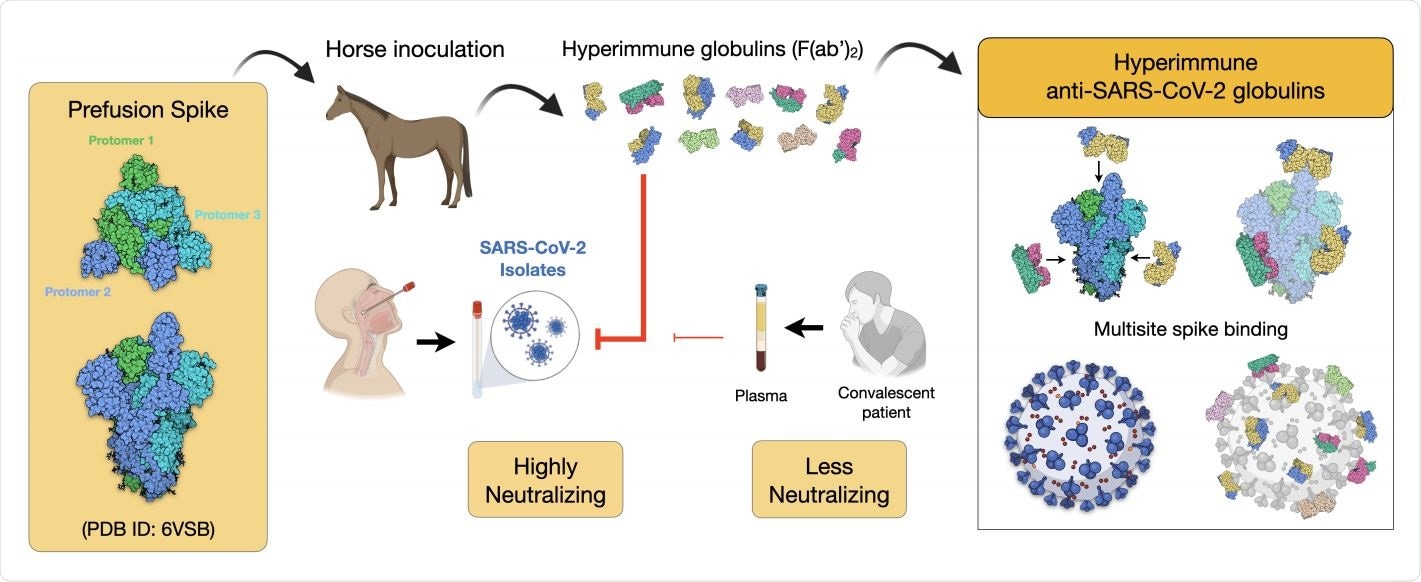
Coronavirus Vaccine Hyper Immune Response
Paris afp two covid 19 vaccine candidates have proven safe for humans and produced strong immune reactions among patients involved in two separate clinical trials doctors said monday.
Coronavirus vaccine hyper immune response. Responses to the b victoria vaccine component were similar across vaccines with the exception of higher post vaccination antibodies among those who received high dose vaccine. The rapid genomic sequencing and open access data together with advanced vaccine technology are expec. Early results show t cell responses may outlast the initial antibody response which could have a significant impact on covid vaccine development and immunity research said dr ladhani. Severe acute respiratory syndrome sars emerged in china in 2002 and spread to other countries before brought under control.
Immune cells for common cold may recognize sars cov 2 experimental coronavirus vaccine is safe and produces immune response sars cov 2 may use key carbohydrate to infect cells. Potent antibodies found in people recovered from covid 19. Evaluations of an inactivated whole virus vaccine in ferrets and nonhuman primates and a virus like particle vaccine in mice induced. As the world is witnessing the epidemic of covid 19 a disease caused by a novel coronavirus sars cov 2 emerging genetics and clinical evidences suggest a similar path to those of sars and mers.
The inoculation produced protective antibodies and t. The covid 19 vaccine being developed by the university of oxford produces an immune response in both elderly and young people and adverse reactions were lower among the elderly british drug maker. The first trial among more than a thousand adults in britain found that the vaccine induced strong antibody and t cell immune responses against the novel coronavirus. The coronavirus vaccine jointly developed by astrazeneca and oxford university has induced a strong immune response in older age groups.
Optimism was raised in july when scientists from oxford university reported their vaccine candidate induced strong antibody and t cell immune responses up to day 56 of the ongoing trial. Because of a concern for reemergence or a deliberate release of the sars coronavirus vaccine development was initiated. Astrazeneca says its coronavirus vaccine triggers immune response among adults published mon oct 26 2020 6 06 am edt updated tue oct 27 2020 5 40 pm edt sam meredith smeredith19.