Coronavirus Vaccine Clinical Trials Update
Pfizer starts phase 3 trial as.
Coronavirus vaccine clinical trials update. These landscape documents have been prepared by the world health organization who for information purposes only concerning the 2019 2020 global of the novel coronavirus. It is also funding the production and research for covid 19 vaccines. A fourth phase 3 clinical trial evaluating an investigational vaccine for coronavirus disease 2019 covid 19 has begun enrolling adult volunteers. Coronavirus vaccine johnson johnson and eli lilly covid 19 vaccine update.
3 november 2020 publication. Results of the clinical trials of both the astrazeneca vaccine and sputnik v vaccine are expected early next year. Known as sovereign 2 it contains the rbd part of the coronavirus. In vaccine news brazilian health regulator anvisa has approved the resumption of human clinical trials of johnson johnson s j j experimental coronavirus vaccine.
With a number of drug companies pressing ahead with clinical trials. Coronavirus vaccine update phase 3 human clinical trial of oxford vaccine begins in pune we have started the phase iii trials of the vaccine candidate covishield. The trial is designed to evaluate if the investigational janssen covid 19 vaccine jnj 78436725 can prevent symptomatic covid 19 after a single dose regimen. Apart from the two indigenous coronavirus vaccines india is also doing the clinical trials of two global frontrunner vaccines the russian sputnik v and the oxford astrazeneca coronavirus vaccine.
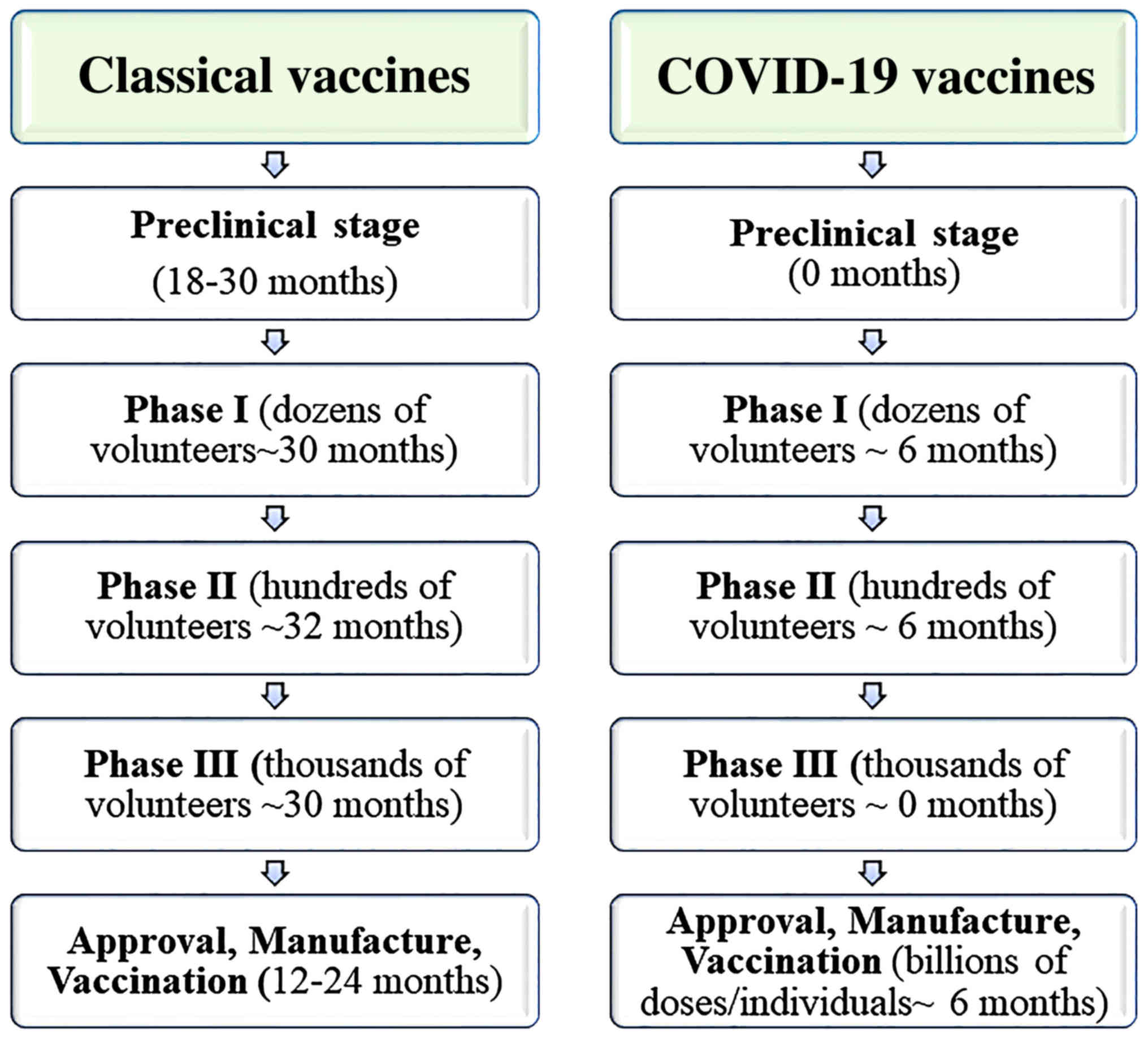
Draft landscape of covid 19 candidate vaccines. The serum institute of india which is sponsoring mid and late stage human clinical trials for the vaccine candidate in india also halted trials a day later. In october cuba s finlay vaccine institute launched clinical trials on their second experimental vaccine for the coronavirus. This tracker lists covid 19 vaccine candidates currently in phase 1 3 trials as well as major candidates in pre clinical stages of.





.jpg?sfvrsn=2da16d60_0)